
Prion-like phenomena have also been described in budding yeast and other fungi. Highly resistant to denaturation and proteolysis, PrP Sc is infectious and templates the conformational conversion of PrP C molecules ( Caughey et al., 2009). Native PrP (PrP C) undergoes a dramatic change in conformation upon conversion to its prion form (PrP Sc), forming distinctive cross-β aggregates termed as amyloid ( Diaz-Espinoza and Soto, 2012). Specifically, the prion form of a protein known as PrP is the causative agent of the TSEs, which afflicts humans and other mammals. Prions are infectious, self-propagating protein aggregates first described in the context of scrapie ( Prusiner, 1982), an example of a class of devastating neurodegenerative diseases known as the transmissible spongiform encephalopathies (TSEs). Furthermore, since both yeast and bacteria form and propagate prions in similar ways, such protein-based inheritance might have evolved in these organisms' common ancestor over two billion years ago. raise the possibility that, even though a prion specific to bacteria has yet to be identified, prions or prion-like proteins might also contribute to the diversity of traits found in bacteria. This propagation depends on a bacterial chaperone protein called ClpB, which is related to another chaperone protein that is required for stable prion propagation in yeast. now reveal that the bacterium Escherichia coli can propagate a yeast prion for over a hundred generations, even when the cells can no longer make the protein that serves as the trigger for the initial conversion. However, it was not known if bacterial cells could support the stable propagation of prions if the initial trigger for prion conversion was removed. The bacteria were engineered to produce two yeast prion proteins-one of which spontaneously formed aggregates that were needed to trigger the conversion of the other to its prion form. Previous work has shown that bacterial cells can also support the formation of prion-like aggregates. Although prions can form spontaneously in yeast cells, their stable propagation depends on so-called chaperone proteins that help to remodel the prion aggregates. These prions, however, do not cause disease or cell death instead, yeast prions act as protein-based elements that can be inherited over multiple generations and which provide the yeast with new traits or characteristics. Prions also form in yeast and other fungi. Bovine spongiform encephalopathy (also commonly known as BSE or ‘mad cow disease’) is an example of a prion disease that affects cattle and can be transmitted to humans by eating infected meat. Prion diseases are well known in mammals, where the prion aggregates typically destroy tissue within the brain or nervous system. As more prions are formed, they then trigger other protein molecules to misfold and join the aggregates, and the aggregates continue to grow and spread within the infected organism causing tissue damage and cell death. Within the aggregate, the prion proteins have a corrugated structure that allows them to stack together tightly, which in turn makes the aggregates very stable. Although they are not living organisms, these prion aggregates can self-propagate when they enter a healthy organism, they cause existing, correctly folded proteins to adopt the prion fold. Unlike most infectious agents-such as viruses or bacteria-that contain genetic material in the form of DNA or RNA, a prion is simply an aggregate of misfolded proteins. coli suggests that the cytoplasmic milieu in general and a molecular machine in particular are poised to support protein-based heredity in the bacterial domain of life. Our demonstration of ClpB-dependent prion propagation in E. Prion propagation in yeast requires Hsp104 (a ClpB ortholog), and prior studies have come to conflicting conclusions about ClpB's ability to participate in this process.
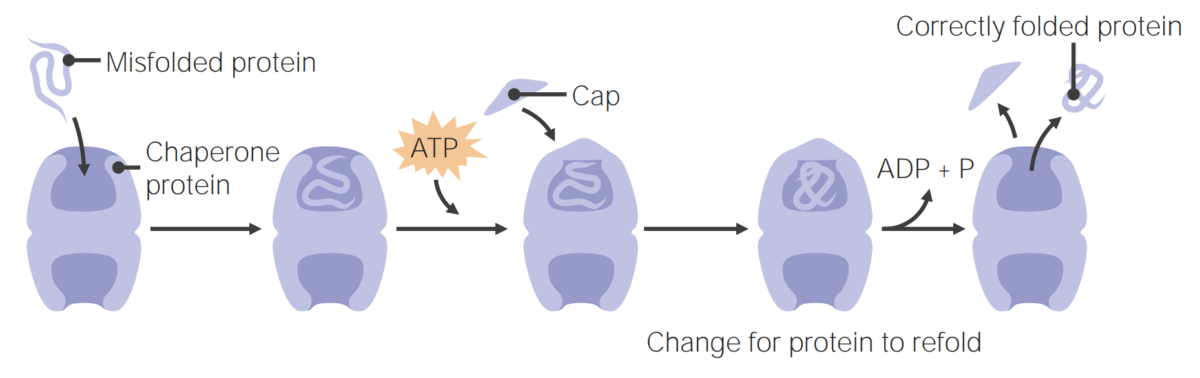

Furthermore, we show that propagation requires the disaggregase activity of the ClpB chaperone. coli can propagate the Sup35 prion under conditions that do not permit its de novo formation. We previously showed that the yeast prion protein Sup35 can access the prion conformation in Escherichia coli. Prions have also been uncovered in fungi, where they act as heritable, protein-based genetic elements. In mammals, the PrP protein can form a prion that causes the fatal transmissible spongiform encephalopathies. Prions are self-propagating protein aggregates that are characteristically transmissible.


 0 kommentar(er)
0 kommentar(er)
